Through Thick and Thin
Winter
2014
Building Blocks - Undergraduate Research and Outreach Projects
Through Thick and Thin
A Study of Metal Oxides
By:Zoey Warecki, Class of 2015, Towson University, MD
Towson University

My interest in perovskite metal oxides started with their potential applications in renewable energy devices such as photovoltaics and photoelectrochemical cells. Made of rare earth elements and alkaline and/or transition metals, the materials could contribute to the clean energy revolution and reduce the world’s current dependence on harmful and limited resources.
Another reason to be interested in the oxides is that they have a reputation for being unpredictable. Will a perovskite be a metal or an insulator? Magnetic or not? They seem to have trouble making up their minds because their properties are extremely sensitive to variations in structure and composition.
When prepared as thin films or other nanostructures, for instance, perovskites display behaviors not observed in their bulk form. Some perovskite thin films possess colossal magnetoresistance, which means they drastically change their resistance to the flow of electricity in the presence of a magnetic field.
Our specific project was born out of a desire to fabricate thin films of calcium manganese oxide (CMO) with low resistance. CMO, like other perovskites, is in bulk an insulator with such high resistance that it is sometimes inconvenient or nearly impossible to accurately measure. Thin CMO films, on the other hand, have measurable resistances and can be fabricated with various deposition methods.
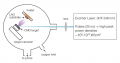
Each of the many deposition techniques for making a thin film in a laboratory has its advantages. Some are cheaper or quicker; others are more reliable or scalable. One of the coolest methods with one of the coolest names is pulsed laser deposition (PLD). It is an expensive method, but it allows the fabrication of thin films of materials with complex structures, such as CaMnO3. The thickness of a sample made with PLD can range from hundreds of nanometers to a few atoms.
We began making CMO thin films with PLD and found that the resistance of the film depended on its thickness. This was due to the difference in size between the crystals in the thin film and the crystals in the substrate on which the film was grown, lanthanum aluminate (LAO). The thinner you make the film, the more CMO has to strain to match its lattice structure to the substrate’s. We then changed our question from “Can we make low-resistance films?” to the more interesting “What role does strain play on these thin films?”
We investigated the size of the unit cells comprising the films and the resistivity of the samples. To measure the dimensions of the cubelike cells, we used x-ray diffraction (XRD), which determines how the planes are arranged in a crystal based on the diffraction angles of x-rays. Then we used a liquid nitrogen cryostat to measure the temperature-dependent resistivities of our samples. These two different measurements, one structural and one electrical, began to hint at the same story: the thinner samples (i.e., the ones with more strain) were more susceptible to the formation of oxygen vacancies, holes in the crystal structure next to oxygen atoms. Understanding how oxygen vacancies change the electrical properties of CMO could help engineers to create new oxygen sensors and other devices.
One particular challenge we faced when first beginning this work was that the resistances of the CMO samples changed over the course of a few days to a week. We needed to organize the experiment in such a way as to minimize any time effects. This seems easy but can often be difficult when equipment, like the XRD, shuts down and needs to be repaired. Experimental physicists must be very patient, or they will become frustrated by interruptions to their work.
I was lucky to have a good advisor who was motivated, passionate, and supportive. He encouraged me every step of the way and pushed me to do good, meaningful work. In my early years of research I made the mistake of not asking questions because I was afraid of looking foolish. I have seen others fall into this dangerous trap. Now I know that fulfilling research starts not with already knowing the answers, but with our ability to ask meaningful questions.
Be Recognized for Your Research
Zoey Warecki received a 2014 SPS Award for Outstanding Undergraduate Research for the work highlighted here. Along with the recognition, she received a free trip to present her research at the 2014 International Conference of Physics Students (ICPS) in Heidelberg, Germany; a $500 honorarium; and a $500 award for her chapter. To read about her ICPS experience and the experience of Helen Meskhidze, who also won a 2014 award, visit www.spsnational.org/programs/awards/2014/OSA/.
The 2015 ICPS will be in Zagreb, Croatia, August 12-19. For more information on the conference and the SPS Award for Outstanding Undergraduate Research, visit www.spsnational.org/programs/awards/student.htm. Applications are due March 16
More from this department
Building Blocks - Undergraduate Research and Outreach Projects