Solving the Clean Water Problem
Spring
2014
Building Blocks - Undergraduate Research and Outreach Projects
Solving the Clean Water Problem
New Nanocomposite Materials and Reactor Systems Developed for Photocatalysis
By:Jeremiah F. Wilson, Sammie Ely III, Bria M. Moore, and Lamont Henderson
Tuskegee University

How clean is your drinking water? It’s a question far from the minds of most US citizens, as we sip from the nearest water fountain. But many countries lack the water filtration technology necessary to obtain contaminant-free water. Solving this important and challenging problem will require new approaches to decontaminating water, such as photocatalysts that utilize the visible light from the sun to degrade toxins.
Photocatalysis is especially attractive for organic pollutant disposal because many toxic organics contain certain chemical structures resistant to traditional chemical attacks. Those structures are preferentially attacked by the reactive oxygen species produced during photocatalysis.[1] Besides oxidation reactions, photocatalysts can reduce chemicals as well, potentially reducing toxic heavy metals and converting CO2 into automotive fuels.[1–3] Some researchers have also experimented with using water-splitting photocatalysis as a means of producing hydrogen, but little success has been achieved for this purpose with visible light.
 Our team of SPS members investigated titanium dioxide (TiO2), an ingredient in cosmetic products (e.g., sunscreen, toothpaste, body powder, lipstick, and soap) that is also widely used in photocatalytic and water-splitting applications because of its high stability, low cost, lack of toxicity, high oxidation potential, and chemically favorable properties. This material can only utilize the ultraviolet portion of the solar spectrum, which results in low total efficiency of catalysis for sunlight energy utilization. Improving the photocatalytic efficiency of TiO2 or developing other photocatalysts with activity shifted toward the visible portion of the solar spectrum would have a significant impact.
Our team of SPS members investigated titanium dioxide (TiO2), an ingredient in cosmetic products (e.g., sunscreen, toothpaste, body powder, lipstick, and soap) that is also widely used in photocatalytic and water-splitting applications because of its high stability, low cost, lack of toxicity, high oxidation potential, and chemically favorable properties. This material can only utilize the ultraviolet portion of the solar spectrum, which results in low total efficiency of catalysis for sunlight energy utilization. Improving the photocatalytic efficiency of TiO2 or developing other photocatalysts with activity shifted toward the visible portion of the solar spectrum would have a significant impact.
Some novel approaches in improving photocatalysts have been successfully implemented and reported in the literature—including doping TiO2 with carbon or nitrogen,[4] coupling nanoparticles to semiconductors,[5–7] and engineering the bandgaps of these materials.[8] In this project, we have developed new nanocomposite materials made of TiO2 combined with indium vanadate (InVO4) via the solid-state ball-milling process, in which metal balls pulverize the materials into fine particles 100–200 nm in size. We have also fabricated a batch-type photocatalytic reactor, a device used for water treatment processes under UV-visible light irradiation.
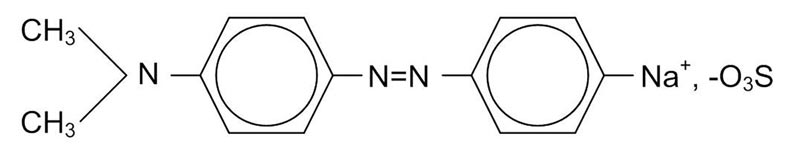
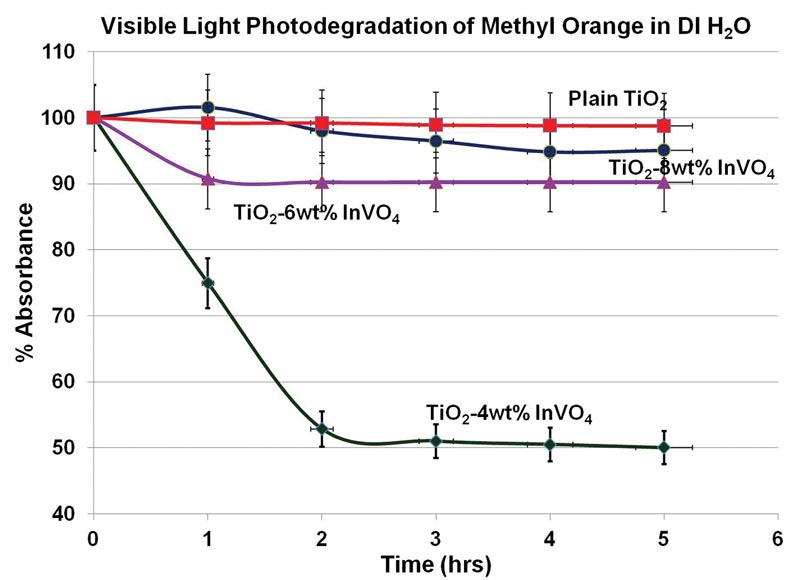
After combining TiO2 with different concentrations of InVO4 (from 2 to 10 percent by weight), we characterized the composites’ surface area, pore size distribution, chemical properties, and morphology. We then mixed each sample with methyl orange (MO), a common industrial dye that cannot be broken down by traditional biological or chemical means; our best composite degraded 50 percent of the toxin in less than 3 hours under only visible light (see Fig. 1). Plain TiO2 degraded no MO. A plausible reason for this enhancement is that the composite nanoparticles have a higher surface area than pristine TiO2, of benefit because the photocatalytic reaction that occurs on the surface enables electron-hole pair separation and recombination to produce reactive oxygen species that in turn degrade the organic chemicals in water.
The first and foremost challenge we overcame during this process was figuring out how to cool the light housing with a water flow; this required designing a  custom water circulation system. One setback we encountered was the loss of product during the ball-milling process; it took trial and error with various proportions of TiO2 and InVO4 to obtain enough samples to complete the experiments, and we had to be very careful to make sure the experimental environment stayed consistent for each sample. We kept detailed notes and observations in log books and encouraged continual communication between ourselves and with faculty mentors.
custom water circulation system. One setback we encountered was the loss of product during the ball-milling process; it took trial and error with various proportions of TiO2 and InVO4 to obtain enough samples to complete the experiments, and we had to be very careful to make sure the experimental environment stayed consistent for each sample. We kept detailed notes and observations in log books and encouraged continual communication between ourselves and with faculty mentors.
While working on this research project, we received hands-on training on several sophisticated and state-of-the-art tools. Literature searches improved our skill at finding articles on Science Direct and other electronic sources crucial for doing background research. By the end of the project, we understood the underlying physics of semiconductor nanoparticles, band energy positions, electron-hole pair separation and recombination, chemical mineralization and intermediate species, materials characterization, and the engineering aspects of photocatalytic reactor design specific to water treatment applications.
We are continuing to optimize our material synthesis process and hope that the addition of other elements, such as nickel, will improve the degradation rate and be a next step toward discovering a solution to our world’s clean drinking water problem. //
This project was funded by a $2,000 Sigma Pi Sigma Undergraduate Research Award. Faculty advisors Sesha Srinivasan and P.C. Sharma oversaw the research.
REFERENCES
[1] J. Herrmann, “Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants,” Catal. Today, 53 (Oct 1999): 115–129.
[2] D.S. Bhatkhande, V.G. Pangarkar, and A.A. Beenackers, “Photocatalytic degradation for environmental applications—A review,” J. Chem. Technol. Biotechnol., 77 (2002): 102–116.
[3] M.R. Hoffmann, S.T. Martin, W. Choi, and D.W. Bahnemann, “Environmental applications of semiconductor photocatalysis,” Chem. Rev., 95 (Jan 1995): 69–96.
[4] K. Madhusudan Reddy, B. Baruwati, M. Jayalakshmi, M. Mohan Rao, and S. V. Manorama, “S-, N- and C-doped titanium dioxide nanoparticles: Synthesis, characterization, and redox charge transfer study,” J. Solid State Chem., 178 (2005): 3352–3358.
[5] S. Srinivasan, J. Wade, and E. Stefanakos, “Visible light photocatalysis via nanocomposite CdS/TiO2 materials,” J. Nanomaterials, DOI 10.1155/JNM/2006/87326 (2006).
[6] S. Srinivasan, J. Wade, and E. Stefanakos, “Synthesis and characterization of photocatalytic TiO2-ZnFe2O4 nanoparticles,” J. Nanomaterials, DOI 10.1155/JNM/2006/45712 (2006).
[7] S.S. Srinivasan, J. Wade, E.K. Stefanakos, and D.Y. Goswami, “Synergistic effects of sulfation and co-doping on the visible light photocatalysis of TiO2,” J. Alloys Compd., 424 (2005): 1–2, 322–326.
[8] N. Kislov, S.S. Srinivasan, Yu. Emirov, and E.K. Stefanakos, “Optical absorption red and blue shifts in ZnFe2O4 nanoparticles,” Mater. Sci. Eng., B, 153 (2008): 70–77.
Drink Deeply
Learn all about the Tuskegee University team’s project at www.spsnational.org/
programs/awards/2013/UGR/index.htm.
Read their recent paper in the Journal of Undergraduate Research in Physics at
www.jurp.org/2014/12030EXR.pdf.
About the Award
SPS supports chapter research activities like this one through Sigma Pi Sigma Undergraduate Research Awards. Each year several awards are made to chapters for amounts up to $2,000. See page 4 for a list of the 2013–14 winners. Find out more and apply online at www.spsnational.org/programs/awards/research.htm.
Acknowledgements
Thank you SPS, AIP, and Sigma Pi Sigma for sponsoring us with an Undergraduate Research Award. We are grateful to Toni Sauncy, Kendra Redmond, Devin Powell, and Tracy Schwab for helping us shape this article.
More from this department
Building Blocks - Undergraduate Research and Outreach Projects
