The Hindenburg Disaster Is a Physics Teaching Opportunity
Fall
2017
Feature
The Hindenburg Disaster Is a Physics Teaching Opportunity
Gregory A. DiLisi, Associate Professor, John Carroll University

This May marked the 80th anniversary of the Hindenburg disaster. On May 6, 1937, the German passenger zeppelin Hindenburg, hovering 300 feet in the air and held aloft by seven million cubic feet of hydrogen gas, burst into flames while preparing to dock at the Naval Air Station in Lakehurst, NJ. The ensuing fire consumed the massive airship in only 35 seconds.
We present the Hindenburg disaster as a case study in the flammability of fabrics. Our goal is to examine the ship’s outer covering and decide whether or not it was the fire’s initial source of fuel. To accomplish this, we piloted a basic vertical flame test with students in an introductory-level undergraduate laboratory. Our test is patterned after the protocol set forth by the American Society for Testing and Materials (ASTM) for determining the flammability of textiles. The case study provides several unique teaching opportunities.
First, we observe the anniversary of this tragedy by bringing it to the attention of a new generation of students, namely, those currently enrolled in our courses. Reexamining a major historical event is a powerful means of piquing students’ interest. Next, we introduce the topic of physics flammability at a level of rigor appropriate for introductory students or more advanced students. Finally, we use this case study to emphasize that scientists no longer adopt a strictly passive approach to history. Instead, scientists now take a forensic approach, bringing sophisticated analytical tools to scrutinize why certain events unfolded. Far from being a set of agreed-upon immutable facts, the historical record is open to reexamination and reinterpretation.
“Reexamining a major historical event is a powerful means of piquing students’ interest.”
“THIS GREAT FLOATING PALACE”
The zeppelin LZ 129 Hindenburg (Luftschiff Zeppelin #129) was launched in 1936 as the premier passenger aircraft of the world’s first airline, the German Airship Transportation Corporation.
The ship was classified as a rigid airship because of its steel frame. Within the steel structure were 16 large gas cells (or bladders) made of gelatinized latex, designed to hold hydrogen gas. The steel structure was covered by panels of cotton cloth doped with various compounds. The flammability of this outer covering plays a pivotal role in the debate surrounding the ship’s destruction.
As the ship approached New Jersey on May 6, 1937, it encountered a storm before reaching the Lakehurst Naval Air Station. After a number of maneuvers designed to get the ship into position at the airfield, the forward grounding lines were dropped. A light rain began to fall. The metal frame was now electrically grounded by the landing lines.
“IT’S BURST INTO FLAMES!”
It’s burst into flames! … and it’s crashing! It’s crashing terrible! Oh, my! …. It’s smoke, and it’s in flames now; and the frame is crashing to the ground, not quite to the mooring mast. Oh, the humanity!
The first sign of trouble appears to have been at the top, rear of the ship, just in front of the vertical fin. Two crew members testified that they noticed a fluttering of the ship’s outer cover at this location—suggesting hydrogen was leaking.1 By 7:25 p.m., a yellow flame appeared on the outside of the ship at this spot. Within seconds, the tail section was engulfed in flames.
Most eyewitnesses described the Hindenburg as burning from the inside out. Within 30 seconds, the entire ship crashed to the ground. In general, passengers and crew in the promenade or public areas of the ship were able to jump to safety while those deeper inside the ship were not. Some family members lived or died based merely on a few feet of separation.
THEORIES
Subsequent investigations by the United States and Germany were inconclusive in determining the cause of the fire. Was it sabotage? No evidence of sabotage was ever found. Was it a lightning strike? Unlikely—the outer covering of the ship had several burn holes, some as large as five centimeters in diameter, proving the ship had survived in-flight lightning strikes during its first year of service.2
Today, a reexamination of the evidence leaves us with two competing theories. Here is what they agree on: As the Hindenburg passed through the storm off the New Jersey coast, it became electrically charged. When the landing lines touched the ground prior to docking, they “earthed” the Hindenburg’s steel frame but not every panel of the ship’s fabric covering. A spark between the charged panel of fabric and the grounded steel frame ignited some source of fuel. The difference between the two theories lies in identifying that source of fuel.
The most likely explanation of events is that the electrostatic discharge ignited leaking hydrogen gas. However, in 1997, engineer Addison Bain put forth the idea that at least early in the fire, the ship’s outer covering itself was the primary source of fuel for the fire.3 The cotton cloth that covered the ship was doped with different mixtures based on cellulose acetate butyrate (CAB), the base resin for what are commonly called lacquers. These coatings were used to keep the outer skin taut for aerodynamic purposes as well as to protect it from wind, water, and small objects. This “Incendiary Paint Theory” (IPT) has merit for two reasons: (i) Hydrogen burns with an invisible flame, yet the Hindenburg was consumed in an enormous yellow and red fireball. One might conclude that something other than hydrogen was burning. (ii) The ship held its position for a few seconds before the stern crashed to the ground. One might conclude that the gas cells were intact when the fire started.
COMBINING PHYSICS AND HISTORY IN THE LABORATORY
We used the IPT as the basis of a new, inexpensive lab activity focusing on the flammability of fabrics. Our activity is modeled after the vertical flame test, ASTM D 6413–99, which has been adopted as an accepted federal test standard.4
We started by presenting students with the historical background information described in the previous sections. Next, we emphasized two concepts: (i) The goal of our activity is not to prove or disprove the IPT, but to showcase how physics can be used in the real world, and (ii) even though our activity focuses only on a vertical flame test, it gives us a quantitative understanding of how flammability is tested and how results can be used to unravel the Hindenburg disaster.
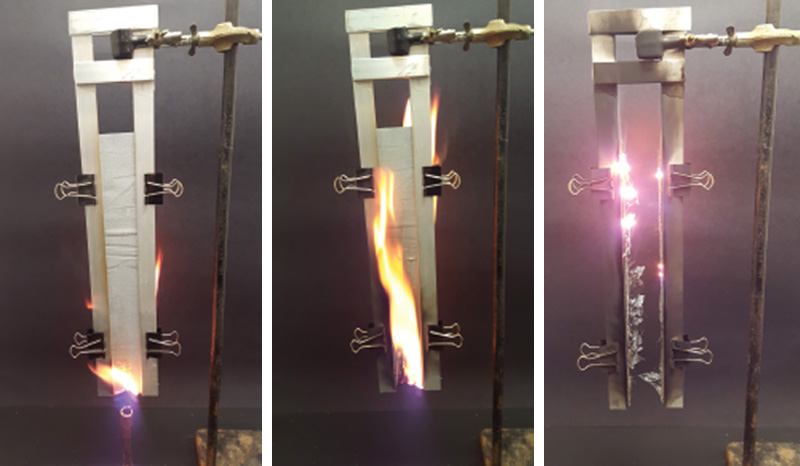
Sample preparation: We chose to test fabrics that were easy to make and inexpensive to buy, yet nicely approximate the outer coverings of the airship. To create the fabric that approximates the covering on the upper portion of the ship, we rolled cotton swatches with a layer of clear lacquer, then a layer of black primer with iron oxide as its tinting agent, then three layers of aluminum resin paste (Genesis LV 1060, purchased at Sherwin-Williams). To create the fabric that approximates the covering on the lower portion of the ship, we rolled cotton swatches with a layer of clear lacquer, then three layers of aluminum paste. We used uncoated cotton swatches as a control. Students trimmed each of these three samples into five strips (12 in × 3 in). Each strip was placed into a frame of sheet metal that secured the strip on two sides, leaving the bottom edge exposed. The frame was clamped together at four locations and suspended in a laboratory hood.
Testing: Each strip was tested and the average of five strips was reported per fabric. A Bunsen burner, with 10-mm inside diameter barrel, was used to create a 1.5-in-high 99%-pure methane flame. The flame was applied for 12 ± 0.25 s (flame-to-strip), as measured by a stopwatch. Students filmed each trial using cell phone cameras in “slow motion” mode [Fig. 1(a)]. Once the flame was removed, students continued to film the strip until any visual flame or glow self-extinguished [Fig. 1(b) and 1(c)].
Analysis: Using their video clips and a ruler, students determined the “afterflame,” the time a visible flame remained on the strip; the “afterglow,” the time a visible glow remained on the strip; and the “char length,” the distance from the edge of the strip to the furthest point of damage. They then calculated the burn rate (char length divided by afterflame) and extrapolated the time needed to burn a 106.1-foot-long swatch of the strip (1/4 of the Hindenburg’s maximum circumference) and the total burn time by adding the vertical burn times for the fabrics covering the lower and upper halves of the ship.
Results: Results support the notion that leaking hydrogen, and not incendiary paint, is the most plausible source of fuel for the fire that consumed the Hindenburg. The outer fabrics just do not burn at a fast enough rate to consume a ship the size of the Hindenburg in a minute. Our dataset shows that a fire would need ~112 minutes to burn a distance roughly equal to the height of the ship, that is to say, from the underside to the topside of the ship.
As a side note, a 2007 episode of the popular show MythBusters (Episode 70 —“The Hindenburg Mystery”) found similar results.5 Although the episode is not peer reviewed and should be viewed with some skepticism, it is a phenomenal visual resource that can be shown to students to emphasize or solidify certain concepts. The episode serves as a good closure activity and can be downloaded from iTunes for $1.99.
CONCLUSION
The 80th anniversary of the Hindenburg disaster presents a compelling case study that brings powerful teaching opportunities to a variety of disciplines. First, the anniversary raises historical awareness in our students while bringing real world applications of physics to them.
Next, the physics of flammability can be treated appropriately at the introductory level since only careful measurements of time, distance, and weight are needed. Our case study can serve as a start-of-the-semester laboratory exercise where safety, measurement, and error analysis are emphasized. Conversely, our case study can also serve as a capstone project in a senior-level engineering course.
Third, the resulting analysis shows students that just as scientific theories are open to reexamination in the light of new or confounding observations, so too are historical events open to revisitation and scrutiny.
ACKNOWLEDGMENTS
The author acknowledges James DeLuca, chemist extraordinaire, for his insights into sample preparations. The author also acknowledges the reviewers of this manuscript, who significantly strengthened the presentation of this work.
_______________________________
1. Bureau of Air Commerce, Air Commerce Bulletin of August 15, 1937, Vol. 9, No. 2 (United States Department of Commerce, Washington, DC, 1937), pp. 28–29.
2. R. Archbold, Hindenburg: An Illustrated History (Warner Books, New York, 1994).
3. A. Bain, “Colorless, nonradiant, blameless: A Hindenburg disaster study,” Gasbag J./Aerostation 39 (March 1999).
4. American Society for Testing and Materials, ASTM D 6413-99, Standard Test Method for Flame Resistance of Textiles (Vertical Test), Annual Book of ASTM Standards (ASTM, West Conshohocken, PA, 2016).
5. “MythBusters: Hindenburg MiniMyth.” Retrieved from http://www.discovery.com/tv-shows/mythbusters/videos/Hindenburg-minimyth/ on Jan. 3, 2017.
_______________________________
This paper, edited and condensed for space reasons, originally appeared in The Physics Teacher 55, 293 (2017). Copyright 2017, the American Association of Physics Teachers. http://dx.doi.org/10.1119/1.4981031
