Mystery Box
Summer
2017
Feature
Mystery Box
This demo for younger students replicates the famous gold foil experiment first conducted by Ernest Rutherford to study the nuclei of atoms. This experiment makes it possible to learn about atomic structure at the macroscopic scale.
MATERIALS:
Cardboard box, a ramp with multiple tracks, marbles, paper, Sharpie markers, and objects that fit under a box, such as: a small rectangular object like a deck of cards, a round object like a mug, something hollow sounding, and something long and narrow.
INSTRUCTIONS:
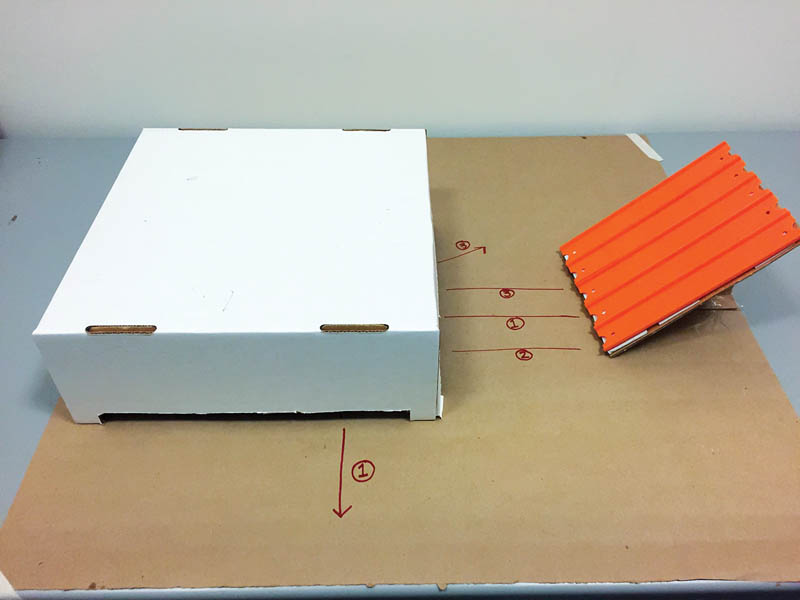
Cut a rectangle out of each side of the box as seen in Figure 1. Now the box has four “legs” and objects can pass underneath. Hide an object under the box.
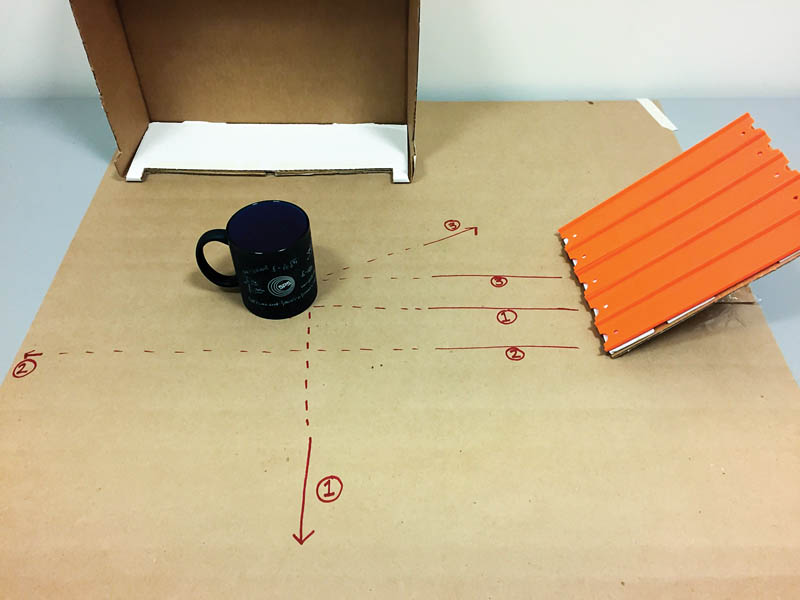
Set up the ramp so it leads into the box as shown in Figure 2. Ask students to roll marbles down the ramp and record (sketch) their trajectories. Have students mark each marble's path with a Sharpie. See if the students can guess what's in the box based on the marble paths! After the students have guessed, you can lift the box to reveal the object.
This experiment simulates an experiment done by E. Rutherford1,2: By firing a beam of alpha particles at a gold foil sheet, he concluded that there were tiny nuclei in atoms.

EXPLANATION:
The object in the box represents the interior of an atom. Just as an atomic nucleus is too small for us to see, we can't see inside the box (until the lid is lifted up), but we can make educated guesses about the contents of the box by observing what happens to the marbles. By firing particles (marbles) at the atom (box) and tracing the pathways, we can learn about the interior of the atom.
COMMON QUESTIONS TO EXPECT:
Why can't we just look inside the box to get the “right” answer?
Sometimes in physics we study things that are very small, and we can't “just look inside” to get the right answer. In other words, there's no peeking in real physics! Even today, with a hundred years of technology, we still can't “see” the building blocks that make up atomic nuclei. So we have to get creative and come up with ways to learn “what's inside the box” without looking.
PHYSICS:
At the beginning of the 20th century, the current atomic model was J. J. Thomson’s “plum pudding” model. He theorized, “Atoms of the elements consist of a number of negatively electrified corpuscles enclosed in a sphere of uniform positive electrification.” His corpuscles (or electrons) were like raisins in a pudding or sea of positive charge.
According to the popular plum pudding model, the alpha particles should have experienced only small angle deflections from the corpuscles. However, the experimental results were that a small percentage (only 1 in 20,000!) of the alpha particles were deflected at large angles, some even greater than 90°.1,2 Since a small fraction are deflected, we know the nucleus must be very small (~2 fm).
Rutherford commented on the results:
It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. . . . When I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive center, carrying a charge.
Thus Rutherford was able to definitively reject the plum pudding model in his 1911 paper3 on his results. Instead he hypothesized the existence of a “central charge” where almost all of the atom’s mass was concentrated, which later became known as the nucleus.
LEARN MORE:
http://chemed.chem.purdue.edu/genchem/history/gold.html
References
- K. S. Krane, Modern Physics, 3rd ed. (Wiley, USA, 2012).
- S. Thornton and A. Rex, Modern Physics for Scientists and Engineers, 4th ed. (Cengage Learning, 2013).
- E. Rutherford, “The Scattering of α and β Particles by Matter and the Structure of the Atom,” Philosophical Magazine, series 6, vol. 21, May 1911, pp. 669-688.
